 Congress has mandated that the Centers for Medicare and Medicaid Services (CMS) move forward with quality-based programs and associated payment models. In 2015, the Medicare Access and CHIP Reauthorization Act (MACRA) was signed into law, expanding the Medicare quality-reporting programs that began as a voluntary incentive ten years ago with the original Physician Quality Reporting Initiative (PQRI). Today it is more important than ever to embrace and maximize success in these programs and be ready to move ahead as they evolve.
Congress has mandated that the Centers for Medicare and Medicaid Services (CMS) move forward with quality-based programs and associated payment models. In 2015, the Medicare Access and CHIP Reauthorization Act (MACRA) was signed into law, expanding the Medicare quality-reporting programs that began as a voluntary incentive ten years ago with the original Physician Quality Reporting Initiative (PQRI). Today it is more important than ever to embrace and maximize success in these programs and be ready to move ahead as they evolve.
Historical Perspective
In 2006, CMS launched PQRI as a voluntary program that would earn participants a bonus payment of 2% of their prior-year Medicare reimbursements. This was seen as a way to collect meaningful data that would lead to improved patient care and optimal patient outcomes, as well as to identify and reduce the costs of providing healthcare. Along the way, the name was changed to the Physician Quality Reporting System (PQRS) and the incentive became a penalty. Medicare payments, whether for primary, secondary or Railroad Medicare coverage, were reduced for the first time in 2015 by 1.5% for those physicians who did not successfully report PQRS data in 2013.
The Value-Based Payment Modifier (VM) was added to the quality-based payment model beginning in 2015 for the largest practices, and in 2017 it will to be applied to Medicare payments to all physicians. In 2018 it will be expanded to include all other Eligible Providers (EPs), including non-physician EPs who bill under their own National Provider Identifier (NPI), based on 2016 reporting. Under both PQRS and VM, the fee schedule is adjusted for reimbursement of services rendered two years following the measurement period. The VM program uses data collected under PQRS to calculate payment modifications that are upward, downward or neutral based on both the quality and cost of care delivery.
Since a negative VM adjustment of up to 4% will be calculated for EPs who do not successfully report under PQRS, and this is added to the 2% PQRS penalty for not reporting, the total impact for not successfully reporting data can be up to 6% of Medicare payments! This is why we emphasize the importance of collecting and reporting PQRS data.
A positive, upward adjustment of Medicare payments is also possible for those EPs who achieve either high quality or low cost, coupled with at least average cost or quality, respectively. In the first year the VM was applied, EPs with low cost and high quality earned an additional 9.78% over the basic Medicare fee schedule! For more information about the VM payment adjustments, review our articles An Overview of the Medical Quality Reporting Provisions Affecting Radiology Practice Reimbursements.
Where are we headed?
The MACRA law expanded these two current programs and aligned them with the Electronic Health Records Meaningful Use incentive program and the Accountable Care Organization (ACO) program to create a single, new Merit-Based Incentive Payment System (MIPS). Under MIPS, beginning with payments in 2019, negative fee schedule adjustments will begin at 4% and rise to 9% by payment year 2022. Additional incentive payments, over and above the VM-like upward adjustment, will be available for “exceptional performance”. The first performance measurement year will be 2017, but the system’s final form is not yet defined. Also unknown, but expected by experts, is the migration of non-government healthcare payers to adopt quality care payment systems based on the CMS model.
What to do in 2016...
For now, participation in PQRS is a must in order to avoid penalties and maximize reimbursement under VM. PQRS is, and will continue to be, the foundation for future CMS pay-for-performance initiatives. Successful participation in 2016 will determine payment adjustments in 2018 as shown here:
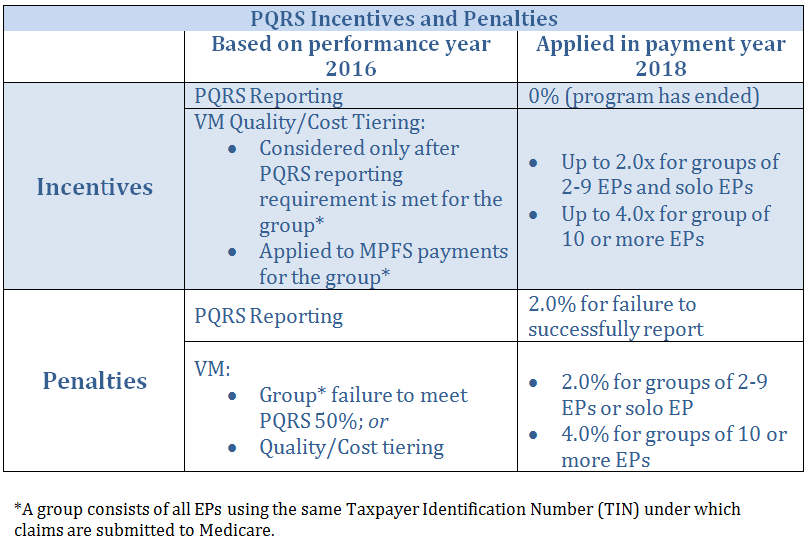
Although the value ‘x’ for the upward adjustments will remain unknown until all of the data is accumulated and evaluated, the downward adjustments for failure to successfully report are fixed: 4.0% for groups of 2-9 EPs or solo EPs, or 6.0% for groups of 10 or more EPs.
Successful reporting in 2016 means reporting on 9 measures under 3 National Quality Strategy (NQS) domains for 50% of patient visits, or one measures group. If 9 measures and/or 3 NQS domains are not applicable, then you must report on as many as possible/applicable to your practice up to these thresholds.
Applicable Measures for Both Claims-Based and Registry Reporting
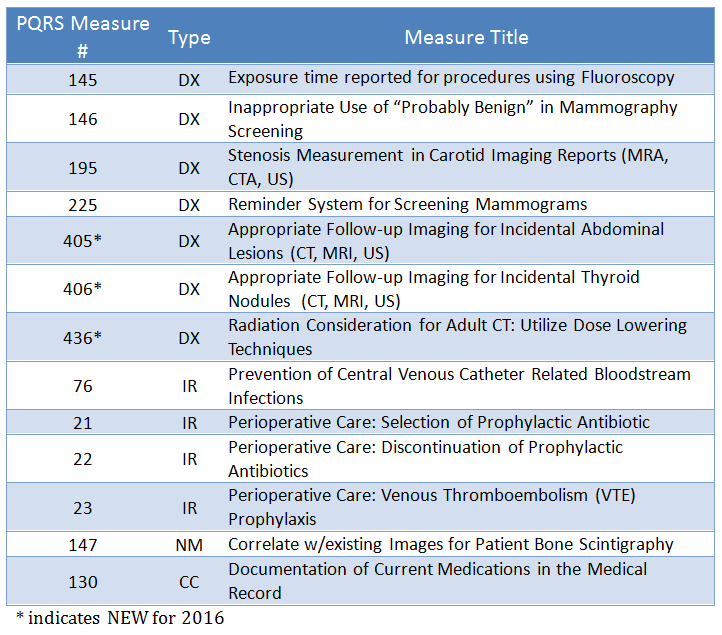
For 2016, there are thirteen PQRS measures for radiologists to choose from that apply to both claims-based and Registry reporting including 3 that are new this year. Of these, 7 are related to diagnostic radiology (DX), 4 are for interventional radiology (IR), 1 is nuclear medicine (NM) and 1 is cross-cutting (CC) for evaluation and management services.
Applicable Measures Only Available in Registry Reporting
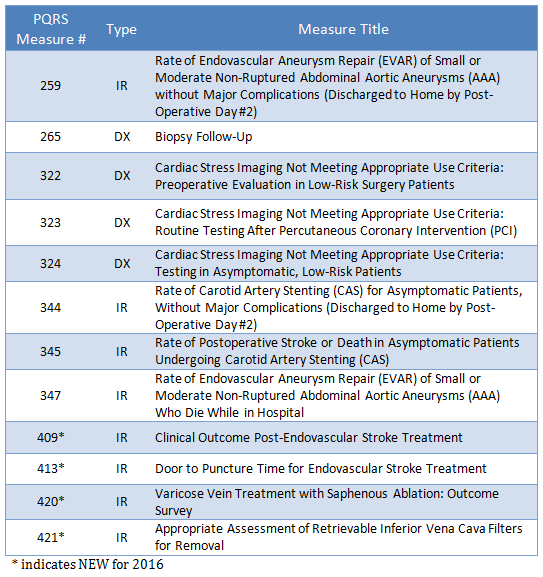
There are 12 additional Registry-only PQRS measures potentially applicable to Radiology. If a provider or group chooses to report through a Registry, they are responsible to report on all applicable Registry measures in order to meet the required 9 measures across 3 Clinical Quality Domains.
Many of these measures are very specific to radiologist sub-specialization, and several new measures are included:
Documentation Tips
The imaging consultation report should always include information that supports application of the relevant measure code for PQRS reporting purposes. The three new measures available for both claims-based and Registry reporting for 2016 will initially be unfamiliar to physicians, and it might be useful for practices to build reminders into their dictation templating system. When considering the documentation of incidental findings, radiologists should always put clinical considerations at the forefront of their decision process. Technologists’ notes that include relevant patient history and are readily available to the radiologist during dictation can provide the information needed to document the reason for follow-up imaging.
Here are some ideas for compliance with the newest measures:
405: Appropriate follow-up for incidental abdominal finding
The goal of this measure is to reduce unnecessary follow-up imaging of incidental lesions without a medical justification. It applies to all of the following found incidentally in asymptomatic patients on an abdominal CT, MR or ultrasound:
- Liver lesion less than or equal to 0.5 cm
- Cystic kidney lesion less than 1.0 cm
- Adrenal lesion less than or equal to 1.0 cm
Documentation must include reference to the incidental lesion(s) if found and state whether:
- No follow-up imaging recommended
- Follow-up imaging is recommended and state medical reason (e.g. known malignancy that could metastasize)
- Follow-up imaging is recommended, without giving medical reason
If no incidental lesions are noted, the study will be coded using an exclusion PQRS code for this measure. Ideally, any time follow-up on these types of lesions is recommended, a medical reason should be stated. A high volume of recommendations without medical justification will result in poor performance in the quality component of the PQRS Value Modifier calculation, which could result in lower reimbursement.
Here are some examples of phrases that might be included in imaging reports:
- “No lesions that need follow-up are noted.”
- “An incidental 0.7cm cystic lesion is noted in the right kidney. No imaging follow-up is recommended unless clinically warranted.”
- “A 0.9 cm lesion is seen on the right adrenal gland. Follow-up imaging is recommended due to the patient’s history of _____”
406: Appropriate follow-up for incidental thyroid nodules
This measure applies to CT or MR of the chest as well as CT, MR or ultrasound of the neck. When an incidental thyroid nodule of less than 1.0 cm is documented in the report for a patient with no known thyroid disease, the imaging report must state one of the following:
- No follow-up imaging recommended
- Follow-up imaging is recommended and state medical reason (e.g. multiple endocrine neoplasia, cervical lymphadenopathy)
- Follow-up imaging is recommended, without giving medical reason
In patients where no thyroid nodule is found the exam will be coded using an exclusion PQRS code for the measure. Failure to state one of the above options will negatively impact your PQRS performance. As with measure 405, the goal is to decrease unnecessary imaging and so the recommendation for follow-up with no reason stated will result in a poor performance score in the PQRS Value Modifier calculation.
Here are some examples of phrases to include in imaging reports:
- “An incidental 0.6 cm nodule is noted in the right lobe of the thyroid. Due to the presence of cervical lymphadenopathy, further imaging follow-up is warranted.”
- “A small (0.5 cm) nodule is incidentally noted in the left lobe of the thyroid. This is of doubtful clinical significance and no imaging follow-up is recommended.”
436: Utilization of CT dose lowering technique
This measure applies to all CT studies for patients age 18 or older. The imaging report must indicate that one or more of the following dose reduction techniques was used:
- Automated exposure control
- Adjustment of the mA and/or kV according to patient size
- Use of iterative reconstruction technique
This measure can be met without documentation in each individual CT report if the practice has a signed protocol document on file attesting that the dose-lowering techniques are in use routinely.
Note that it is possible for Measure 436 to be used in conjunction with some other measure. For example, when a CT exam that uses dose-lowering techniques reveals an incidental finding that is described in Measure 405 or 406, the use of both measures would be appropriate.
Conclusion
Radiologists gained some PQRS measures to use for 2016 and this increased selection will help them meet the requirement for successful reporting of 9 measures for 50% of patient visits. Adequate documentation in the imaging report is essential for successful reporting. Failure to achieve success in 2016 will result in penalties of up to 6% of Medicare reimbursement in 2018, although achieving high quality or low cost coupled with at least average cost or quality can earn significant additional reimbursement above the baseline Medicare fee schedule. Practices must adopt policies and procedures to assure compliance with Medicare’s quality reporting programs in whatever form they take, as this method of payment modification will be a part of the system into the future.




